The reactions of addition are important actions with regard to organic syntheses, because through them an interesting number of organic substances can be developed.
For the addition reaction to occur, it is necessary to create two binding sites in the carbon chain, which occurs simply when the chain is unsaturated (presence of pi link). Thus, when the pi link is broken, the sites appear in the chain.
A group of substances that can be used in addition syntheses are cycloalkanes or cyclans, group of hydrocarbons with cyclic and saturated chains. They escape the general rule of addition as they do not have a pi link, but depending on some conditions (heat, catalyst metal, acid medium) they can undergo a chain break between two carbon atoms, causing two binding sites to appear for the addition to take place. However, there is a fact that restricts the use of these compounds. The fact is the call ring stress theory, proposed by Adolf V. Bayer in 1885.
For the sigma bond between the carbons to be broken, there must be a certain instability between the carbons. This instability is related to the angle of bonds between carbons. According to Bayer, the angle that provides a
great stability for the sigma link is 109.47O. Thus, chains that have an angle between carbons smaller than 109.47O they tend to have instability in the sigma bonds between the carbons that form the chains, which favors their breakage allied to an ideal external condition.The only cycloalkanes that have an angle smaller than 109.47O among its carbons are cyclopropane (60O), cyclobutane (90O) and cyclopentane (108O). Cyclopentane has an angle very close to 109.47O it has very stable sigma bonds, therefore it cannot undergo any addition reaction. Below we have the structures of these three cycloalkanes:
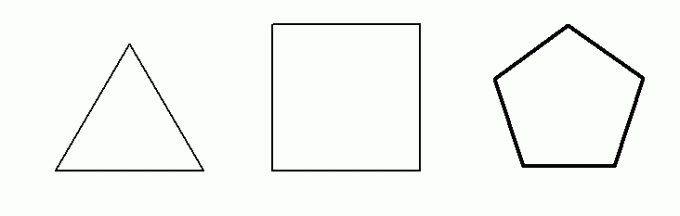
Structural formula of cyclopropane, cyclobutane and cyclopentane, respectively
Observation: Any cycloalkane that has more than 5 carbons in the chain will carry out a substitution reaction for example, and never an addition because the sigma bonds between their carbon atoms are extremely stable.
The addition reactions that can happen with cycloalkanes are basically all, but with cyclopentane the only ones it can undergo are hydrogenation and halogenation. Below are examples of additions with cycloalkanes and their conditions of occurrence:
a) Hydrogenation
Reaction of the cycloalkane with the hydrogen molecule with the presence of powdered metallic catalyst (zinc, nickel or platinum) in the presence of heating (around 180°COÇ). In this reaction, the cycloalkane will give rise to a alkane. In this reaction, after the cycle is broken, one hydrogen atom is added to each of the carbons of the sigma bond that was broken.
Do not stop now... There's more after the advertising ;)
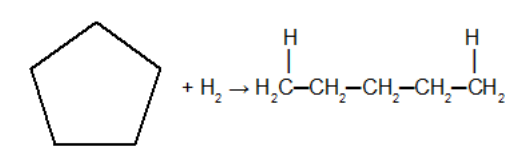
Addition reaction in cyclobutane using hydrogen
b) Halogenation
Reaction of the cycloalkane with the halogen molecule (chlorine, bromine, iodine, for example) with the presence of iron III chloride catalyst (FeCl3). In this reaction, the cycloalkane will give rise to a organic halide with two halogen atoms in the chain. After the cycle is broken we have the addition of a halogen atom on each of the carbons of the sigma bond that was broken.
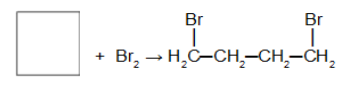
Addition reaction in cyclobutane using bromine
c) Reaction with Halogenhydrides (acid halides)
Reaction of the cycloalkane with the halogen-containing inorganic hydrate molecule (HCl. HBr, HI). As an acid is used as a reactant, a catalyst is not used. In this reaction, the cycloalkane will give rise to a organic halide with only one halogen atom in the chain. After the cycle is broken we have the addition of a hydrogen atom on one of the carbons of the sigma bond that was broken and a halogen on the other carbon. Obeying Markovnikov's rule (H on the most hydrogenated carbon and halogen on the least hydrogenated carbon).
Note: It only occurs with cyclopropane and cyclobutane.

Addition reaction in cyclopropane using hydrochloric acid
Note: It only occurs with cyclopropane and cyclobutane.
d) Hydration
Reaction of cycloalkane with a water molecule in the presence of sulfuric acid and heating. In this reaction, the cycloalkane will give rise to a mono alcohol (alcohol with only one OH group in the chain). After the cycle is broken we have the addition of a hydrogen atom on one of the carbons of the sigma bond that was broken and a hydroxyl (OH) on the other carbon. Obeying Markovnikov's rule (H on the most hydrogenated carbon and hydroxyl on the least hydrogenated carbon).
Note: It only occurs with cyclopropane and cyclobutane.
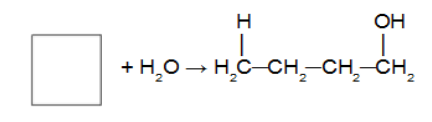
Addition reaction in cyclopropane using hydrochloric acid
By Me. Diogo Lopes Dias
Would you like to reference this text in a school or academic work? Look:
DAYS, Diogo Lopes. "Additions in cyclans"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/adicoes-ciclanos.htm. Accessed on July 27, 2021.
Chemistry

Hydroxyl functional group, Primary alcohols, Secondary alcohols, Tertiary alcohols, Methanol, Glycerol, Ethanol, preparation of nitroglycerin, manufacture of paints, production of alcoholic beverages, acetic acid, fuel for automobiles.

