For a long time the mystery of the origin of fire was the subject of philosophical speculation. Several theories have emerged to explain what happens to materials when they combust.
One of them was developed by the German chemist Georg Ernst Stahl (1660-1734). When he read a book by Johann Joachim Becher (1635-1682), published in Vienna, in 1667, with the title “Physica subterranea”, something caught his attention. In this book, Becher presented his own theory of elements. According to him all substances were composed of three types of land. One of them was the penguin land (literally, “fat earth”), which gave the substance oily qualities and the property of being combustible. In other words, for example, think of a wood that is burned. In the beginning it was composed of ashes and penguin land, at the end of combustion it released the earth and only the ashes remained.

Images by German scientists Johann Joachim Becher and Georg Ernst Stahl (creator of the phlogiston theory)
In reading this book, Stahl gave the
penguin land a new name: "phlogiston”; of Greek origin “phlogios”, which means “fiery”. So, he created a new theory: the “phlogiston theory”; and according to her combustible materials such as paper, wood, sulfur, charcoal and vegetable oils had a common flammable principle present only in combustible materials. If some material didn't burn, it's because it wouldn't have phlogiston in its composition.This theory remained satisfactory for a long time because it explained many of the greatest mysteries of material transformations. In addition to explaining phenomena involving combustion, it also encompassed those related to oxidation. Let's look at two of them:
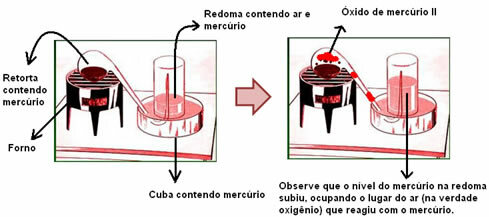
* Without air, combustion does not occur- According to Stahl, the phlogiston needs to go into the air during combustion. But a certain amount of air only contains a part of phlogiston; thus, if we took the air out of the system, combustion would cease because the phlogiston would have nowhere to go. Example: if we place a glass over a lighted candle, it will go out. Furthermore, he indicated air as essential for combustion because it would be the one that would transport the phlogiston from one body to another.
Do not stop now... There's more after the advertising ;)
* Metals increase their mass after burning, corroding or rusting, that is, their oxidation – Phlogiston was repelled by the earth, so the more phlogiston a material had, the lighter it would be. Therefore, when burning, the metal became heavier. Another point that supported his idea was the fact that the oxide has greater mass than the metal; therefore, he concluded that the metal had more phlogiston than the oxide.

Cover image of Becher's book, which Stahl drew upon to create the phlogiston theory
However, this theory was abandoned as some factors contradicted his explanation. For example, paper had less mass after it was burned, unlike metal.
A culmination of the fall of this theory was the fact that in the 18th century, Antoine Laurent Lavoisier (1743-1794) discover, through numerous well-designed and controlled experiments, the importance of a chemical element in the process of combustion. This element was oxygen (O). This is how the phlogiston theory was abandoned.
By Jennifer Fogaça
Graduated in Chemistry
Brazil School Team.
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Theory of Phlogiston"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/teoria-flogistico.htm. Accessed on June 28, 2021.