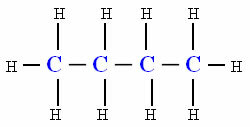
At nitriles, also called cyanides, are a class of organic compounds in which their functional group (__C≡N) is obtained by replacing the hydrogen in the gas cyanide (HCN - hence the name cyanide) by some organic radical. The cyanide gas itself is considered a nitrile.
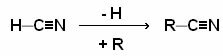
Nomenclature:
The nomenclature of nitriles can be done in two ways:
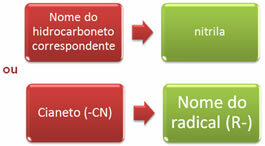
Examples:

Uses, applications and procurement:
Nitriles appear in various parts of nature, see some of these apparitions:
* Pesticide Extraction: Ethanonitrile, better known as acetonitrile, is a solvent widely used in Organic Chemistry to extract pesticides from samples of plants, seeds and soy derivatives. This makes it possible to identify which pesticides were used;
* Animal defense system: the polydesmid is a blind decomposing animal that lives in the remains of vegetables, fruits and meat. It protects itself by producing hydrocyanic acid, which drives away its enemies. Hydrocyanic acid is hydrogen cyanide gas in an aqueous medium, releasing H ions+ and CN-. This last ion is extremely toxic and can kill;
* In seeds and vegetables: seeds of fruits such as peaches, grapes, cherries and apples contain in small amounts a nitrile called amygdalin, whose structure is shown below. In addition, this compound is also present in the leaves and roots of wild cassava. Therefore, when feeding this vegetable to cattle, it is necessary to chop them well and leave them to dry in the sun, so that the HCN evaporates. And when making food for humans it is necessary to cook for a long time;
Do not stop now... There's more after the advertising ;)

*Manufacture of synthetic fabrics: acetonitrile or vinyl cyanide is the nitrile most used for this type of production;
* Metallurgy and metallic electroplating (galvaplasty): cyanide solutions are widely used in industries for these purposes;
* Poisons: many spy or police movies show that capsules containing sodium or potassium cyanide react with the hydrochloric acid in the stomach and cause the person to die from poisoning. A real case of this was that of the Russian monk Rasputin, who, in 1916, suffered an attempt at poisoning by cyanide that was mixed in a pudding. He didn't die because glucose and sucrose combine with cyanide to generate cyanhydrin, which has virtually no toxicity.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Nitrils or Cyanides"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/nitrilas-ou-cianetos.htm. Accessed on June 27, 2021.